Ordered by weight (if set) and creation date.
Research
Research in the Prehoda lab focuses on the biochemical processes that allow cells to respond to changes in their environment. Environmental cues, or “signals”, pass across a cell’s plasma membrane and initiate a molecular program that associates a signal with an appropriate response. For example, during development stem cells respond to signals from neighboring cells by undergoing a series of precise divisions that lead to the multitude of tissues and organs in complex organisms. Our research attempts to uncover the molecular programs that control highly regulated events like these, and elucidate the mechanisms of information transfer that comprise them. In many instances, protein-protein interactions underlie the regulation of complex cellular processes. Because protein-protein interactions are critically important for cellular signaling, many signaling proteins contain specialized domains that are responsible for binding target proteins.
Figure caption: Structures of PDZ domain complexes involved in stem cell division. On the left, the PDZ from the protein Par-6 is bound to a carboxy-terminal sequence (N and C-terminii of the ligand are labeled). This mode of binding is enforced by a steric mechanism – residues that would extend past the c-terminus would clash with residues from the domain itself. On the right, an internal sequence is shown bound to the Par-6 PDZ domain. This sequence bypasses the carboxy-terminal requirement by taking advantage of plasticity in the PDZ domain.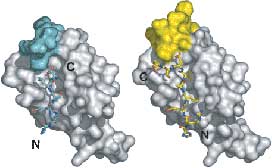
In recent work, we have shown how PDZ protein interaction domains, one of the most common in the human genome, are regulated, and how they specifically bind to carboxy-terminal sequences. We are also studying how interactions between domains in the same protein lead to the complex signaling observed in cells. For example, in the membrane-associated guanylate kinase (MAGUK) family of proteins an intramolecular interaction between an SH3 and GK domain regulates the ability of each domain to bind ligands.
Part of our research program involves determining structures of protein complexes using X-ray crystallography or nuclear magnetic resonance. This structural information is complemented by biochemical, biophysical, and cell biological experiments in order to obtain a complete description of the system under study.
